We ensure that your telecommunications products comply with all technical and regulatory requirements set by ANATEL. From the initial steps to final certification, we manage the entire process, ensuring your products are fully prepared for safe commercialization in the Brazilian market, while adhering to all current legislation.

How ANATEL Approval Works
ANATEL (Brazilian National Telecommunications Agency) certification is a mandatory process for all telecommunications products that will be sold and used in Brazil. This process ensures that devices comply with the technical and regulatory standards required by the Brazilian government, guaranteeing the safety, quality, and compatibility of these products in the national market.
Main Steps of ANATEL Certification:
Why is Homologation Important?
ANATEL homologation is essential to ensure that telecommunications products operate efficiently and safely, without causing interference with other devices and networks. Additionally, it is a mandatory legal requirement for the commercialization of these products in Brazil, protecting both consumers and manufacturers from potential safety and quality issues.
We offer comprehensive support in the INMETRO certification process to ensure that your medical products, home appliances, and IT equipment meet the quality and safety standards required by the Brazilian market. From technical analysis to obtaining the compliance seal, our team oversees every step to ensure your products are regulated and can be safely marketed.
INMETRO (National Institute of Metrology, Quality, and Technology) certification is a mandatory process to ensure that products sold in Brazil meet the quality, safety, and efficiency standards established by the Brazilian government. For electromedical products, household appliances, and IT goods, this certification is essential to ensure that the devices operate safely and efficiently, minimizing risks to the health and integrity of users.
INMETRO has two types of certification: Compulsory and Voluntary.
In both cases, an INMETRO ordinance defines the mandatory requirements that must be followed by all companies producing a specific product, as well as the deadlines for the company to comply with the regulation. The first step is to determine if there is a certification applicable to your product and if the certification is compulsory or voluntary. The list of products covered by INMETRO’s Conformity Assessment Program can be found on the institute’s website at the following links:
Main Steps of INMETRO Certification:
INMETRO accepts test reports conducted abroad by laboratories accredited by ILAC (International Laboratory Accreditation Cooperation) members and the IECEE CB SCHEME (Worldwide System for Conformity Testing and Certification of Electrotechnical Equipment and Components), depending on the type of product. This acceptance allows manufacturers and importers to expedite the certification process in Brazil, as long as the reports comply with Brazilian technical requirements and standards.
Why is INMETRO Certification Important?
INMETRO certification is essential to protect consumers and ensure that electromedical products, household appliances, and IT goods are safe and efficient. Moreover, it is a legal requirement for the commercialization of these products in Brazil, ensuring they comply with international quality and safety standards. This provides manufacturers with greater credibility in the market and gives consumers the confidence that they are purchasing products that meet all safety regulations.
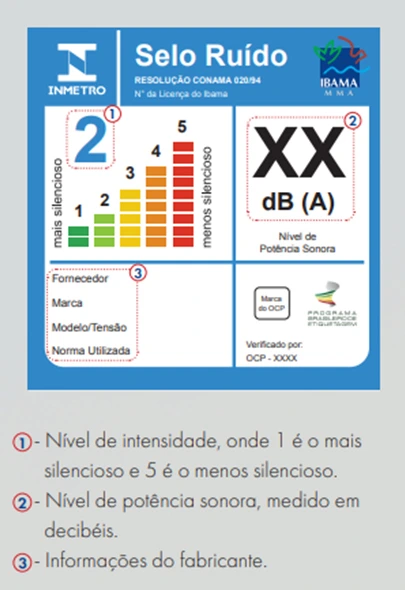
The IBAMA Noise Label is a mandatory certification in Brazil for certain types of home appliances that emit noise, with the purpose of informing consumers about the noise level produced by the device. Created in accordance with the National Program for Education and Control of Noise Pollution, the label is regulated by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA).

The Noise Label serves two main purposes:
How does the IBAMA Noise Label work?
The process of obtaining the label involves the following steps:
Which products require the Noise Label?
The Noise Label is mandatory for a variety of home appliances, especially those that traditionally generate higher levels of noise during use. Examples of products that need this label include:
Importance of the Noise Label
The IBAMA Noise Label is an important tool for both environmental protection and improving consumers’ quality of life. It raises awareness about noise pollution and promotes more sustainable and comfortable choices in everyday life.
The registration of medical products with ANVISA (Brazilian Health Regulatory Agency) is a mandatory process for the commercialization and use of these products in Brazil. ANVISA regulates the entry of medical devices and ensures that these products meet the necessary requirements for quality, safety, and efficacy, protecting public health

The process involves submitting a series of technical and administrative documents, which vary according to the product’s risk class. The main steps include:
The main goal of medical product registration with ANVISA is to ensure that they are safe and effective for use in the healthcare system and by end consumers. By requiring compliance with technical standards and risk assessment, ANVISA protects public health from low-quality products or technical failures that could harm patients. ANVISA also ensures that imported or locally manufactured medical products comply with international standards, promoting competitiveness in the national market and building consumer trust in the available products.
This regulation is essential for ensuring that doctors, hospitals, and the general public have access to reliable and high-quality products.
Our engineering team, with over 10 years of experience, provides comprehensive support in configuring samples for certification testing. With state-of-the-art equipment, such as Spectrum Analyzers for WLAN and CMU200 & CMW500 for WWAN, we ensure that your products are properly configured to meet technical and regulatory requirements. The entire process is carried out in our office, ensuring accuracy, efficiency, and reliability in test results.

Currently, national laboratories are facing a high volume of projects, which compromises their ability to meet demand quickly and with the necessary lead time to configure and verify products. This situation significantly increases the risk of test delays, as if the laboratory is unable to configure the product before the scheduled date, the test may be postponed or even canceled, potentially delaying the project’s timeline by several months.
To mitigate these risks, E3Tech has developed a dedicated structure equipped with high-tech instruments and a team of qualified engineers. This team is responsible for verifying test samples and ensuring they are 100% operational, minimizing the chances of delays and ensuring greater efficiency in the certification process.
We offer professional technical translation services, including certified translations, for manuals, ISO certificates, and other critical documents. Our team of specialized translators guarantees precision and clarity in adapting content into Portuguese (for Brazil) and Spanish (Latin America), ensuring that all specifications and standards are accurately conveyed. This helps to facilitate compliance with regulatory requirements while ensuring the content is easily understood by end users.
Welcome to our informative blog! As a Brazilian leading company in product certification, we specialize in helping foreign companies navigate the complex regulatory environment in Brazil.
With over 20 years of experience, our expertise ensures a smooth process for achieving certification in this dynamic market. Whether you’re looking to understand how to get certified in Brazil or the latest updates on product certification in Brazil, this blog is your go-to resource.
On our blog we are dedicated to providing clear, actionable insights to help you succeed in bringing your products to the Brazilian market with confidence.
Sitemap
About us
E3Tech is a leading Brazilian company in product certification with ANATEL and INMETRO, with over 20 years of experience.